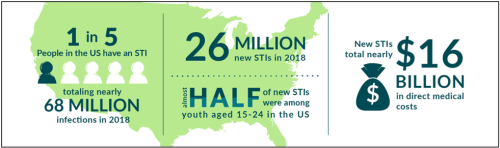
Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (GC) are the two most common bacterial sexually transmitted infections in the United States. The incidence of CT and GC have increased consistently since 2014, and current rates are at an all-time high. CT and GC infections are treatable with antibiotics. However, there is a risk for severe health consequences such as chronic pain, reproductive health complications, and increased risk of HIV transmission when individuals do not get properly diagnosed and treated for these infections. Given the increasing rates of CT and GC infections and the potential consequences, if left untreated, the need for additional screening, testing, and treatment of sexually transmitted infections in at-risk populations is clear. Check out the latest STI rates in your area with Florida Charts.

Despite these concerning increases, many primary care providers do not routinely conduct the sexual history risk assessments needed to determine appropriate testing for CT and GC at every routine monitoring visit. Unless clients have symptoms in non-genital sites that indicate an STI, many providers rely on urine screening alone. However, individuals with CT and GC in the throat and rectum usually have no symptoms, and infections are often localized, which means testing is required at the specific infection location. As a result, it is common for some client populations, such as men who have sex with men, to test positive in throat or rectal specimens but test negative in genital or urine samples.
Clients may feel uncomfortable sharing intimate details about their sexual behaviors to determine which sites to test. They may also be reluctant to consent to a sample collection that they feel is invasive, such as an unanticipated rectal swab! Client-centered interventions are necessary to overcome these hurdles and invite the client to take a more active role in their sexual health. Automated risk assessments and the ability to self-collect any recommended samples may eliminate feelings of shame or embarrassment while serving as an additional opportunity for health education.
Visit the CDC website for more information about national STI rates.
We tested an intervention to reduce stigma and improve bacterial STI screening and testing at a medium-sized Ryan White HIV/AIDS Program funded HIV clinic in Florida. Clients 18 years or older were asked to complete an automated, comprehensive interval sexual history at routine clinic visits between August 2020 and August 2021. Automated sexual history surveys were conducted with audio computer-assisted self-interview (ACASI) software, where each question was read aloud to the client over a headset. The ACASI sexual health screening tool generated an automated summary of bacterial STI tests recommended from the clients' responses to questions regarding symptoms and potential exposures since their last provider visit. Self-collection of recommended CT/GC nucleic acid amplification test (NAAT) specimens was implemented to give the client more control and reduce the burden on the clinical staff of collecting unscheduled or unanticipated specimens. When extragenital site CT/GC testing was recommended, a health educator explained the procedures before providing collection kits. The University of Washington STD Prevention Training Center’s Test Yourself visual guides were posted in clinic restrooms for the client to reference during self-collection. Following each screening, consenting clients completed a client satisfaction survey that asked about their comfort in the clinic and with the self-collection process.
The University of Washington STD Prevention Training Center offers free Test Yourself visual guides and other educational resources.
In total, 223 clinic clients agreed to participate in our evaluation of the intervention. In preliminary analyses, testing for CT/GC returned a combined incidence rate of 10% (n=5) for rectal, 4% (n=4) for throat, 6% (n=2) for vaginal swabs, and 5.3% (n=10) for urine collection. A total of 80 unique participants underwent screening for CT/GC in the throat or rectum at one or more clinic visits. Six of the 9 participants with a positive CT or GC result in rectal or throat specimens had no symptoms prior to testing, and 8/9 tested negative in urine collected at the same visit.
Most clients chose to self-collect their throat and rectal specimens after being taught how, and clients routinely reported ease and convenience with the self-collection process. A total of 31 unique participants self-collected rectal swabs for CT/GC, and 94% (n=29) provided positive feedback regarding their experience. Fifty-eight unique participants self-collected throat swabs, of which 78% (n=45) responded positively in feedback. Participants cited privacy and confidence in the provided training when discussing feeling comfortable self-collecting rectal specimens, and participants who self-collected throat specimens reported that it was easy to do and felt less invasive.

Adding the ACASI sexual history to each client's clinic visit improved STI detection by increasing the number of times per year that at-risk clients were screened, tested, and treated for new and asymptomatic infections. The automated survey design allowed clients to quickly and privately disclose risk encounters that warranted testing in throat and rectal sites while offering additional opportunities for health education. Returning clients that had taken part in the intervention were better informed of the type of sexual encounters for which throat or rectal testing is recommended, and some self-requested throat and rectal screening following risk encounters at subsequent visits.
Our results echo growing evidence that appropriate extragenital site-specific screening plays an essential role in diagnosing CT and GC accurately. Innovative improvements to bacterial STI screening and testing are necessary to combat the record-high rates of bacterial STIs and address antibiotic resistance. However, barriers to implementing these much-needed interventions remain. Insurance providers may limit the number of pathogen-specific tests performed at a given time or limit the number of tests per year covered by a client's policy. Clinicians and staff may face time constraints, feel uncomfortable discussing sexual behaviors, lack the skills to obtain accurate information regarding exposures and risks, or be unaware of CDC recommendations for extragenital screening, testing, and treatment.
Homeless or unstably housed individuals often face barriers to care and treatment due to feelings of shame and stigmatization. Our sample population echoed these concerns, and participants discussed fear of embarrassment if their rectum was not sufficiently clean during specimen collection. Self-collection may aid in reducing barriers for clients at risk for rectal CT and GC. However, laboratories are required to do validity studies of self-collected (versus provider-collected) extragenital site specimens in order to process self-collected extragenital site specimens. Such restrictions to sample processing in laboratories serve as an additional barrier to provider adherence to CDC recommendations for routine STI screening and testing. This barrier has been recently highlighted with COVID-19. Increased telemedicine visits with the use of commercial laboratory community-based specimen collection sites instead of clinic-based lab specimen collection has resulted in clients not being able to be tested at extragenital sites for CT/GC.
As rates of bacterial STIs continue to rise, both in Florida and across the United States, it is evident that new methods for detection and diagnoses are needed. Building self-administered sexual history STI risk assessments into the standard of care at each clinic visit served as both a valuable diagnostic tool and normalized discussions of sexual health between clients and practitioners. The additional privacy afforded by the automated assessment and the ability to self-collect samples offers a client-centered approach to clinical care and allows clients to take ownership over their sexual health. For more information about incorporating these resources into your clinical practice visit the Improving STI Screening and Treatment among People with or at Risk for HIV project resource page on TargetHIV.
